In a fascinating feat of genetic engineering, scientists have successfully grown “mini antlers” on the heads of mice by incorporating deer genes into the mouse genome, offering a glimpse into the regenerative potential hidden within mammals. The groundbreaking research suggests that even in species that have lost the ability to regenerate organs, there may still be regenerative genes waiting to be harnessed for various applications.

Antlers, growing at an astonishing rate of 2.75 centimeters (approximately 1 inch) per day, stand out as one of the fastest-regenerating tissues in the animal kingdom. This unique characteristic provides a valuable opportunity to explore how mammals can regenerate cells regularly. Given that most mammals lack the ability to regenerate organs and many tissues, the annual regrowth of antlers presents an unprecedented insight into the realm of regenerative medicine, particularly for bones.

Delving into the mechanics behind the regrowth of Sika deer antlers, Chinese researcher Toa Qin and collaborators sought to unravel the secrets behind this rapid regeneration. Creating a regenerative “atlas” of Sika deer antlers, the researchers isolated individual cells and genes crucial in the development of antler tissue.
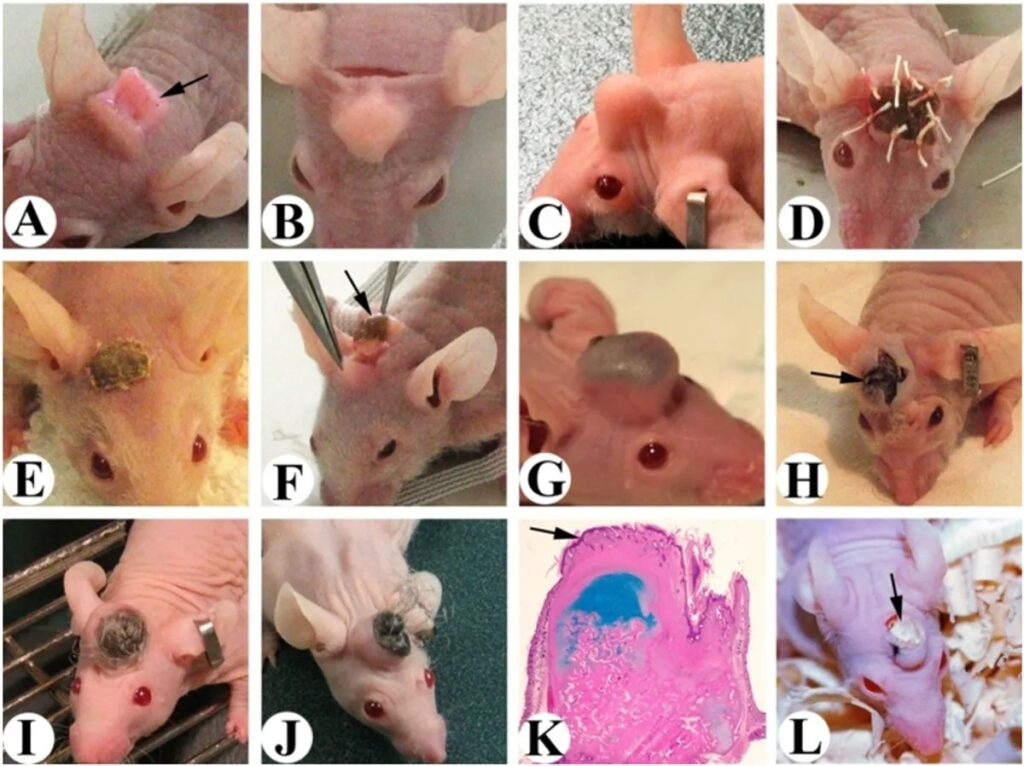
A key revelation emerged ten days before the antlers naturally shed: a highly active type of stem cell that played a crucial role in regeneration. Even after shedding, these cells lingered with the antlers for a short period. By the fifth day post-shedding, a new subtype of stem cells emerged, marking a critical stage in the regenerative process.

Armed with insights from multiple growth stages, the research team selected the stem cells with the highest regrowth potential, originating from antlers shed around five days prior. Cultivating these cells in a Petri dish, they then implanted them into the heads of mice. Remarkably, after just 45 days, the mice sported clearly identifiable mini-antlers. These miniature structures resulted from the stem cells differentiating into osteochondral tissue, a key component in bone fracture repair.
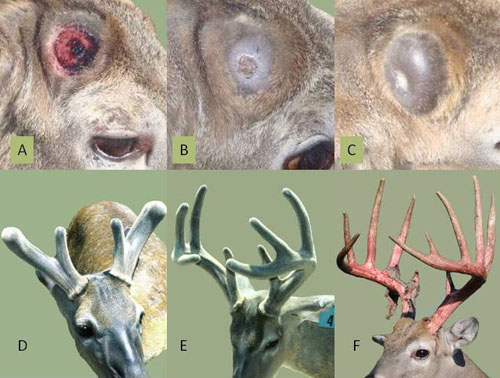
The rapid elongation of these mini-antlers provided researchers with a tangible demonstration of the genetic mechanisms governing their development, offering invaluable insights into their potential applications in human bone medicine. While the direct translation of these findings into repairing broken human limbs may be a distant prospect, the study sheds light on the intricate processes of tissue regeneration within mammals.

However, ethical considerations loom large over the horizon, especially regarding the cross-species implantation of cells. Additionally, significant safety trials would be imperative before such a procedure could be considered for regulatory approval. Nonetheless, unraveling the underlying mechanisms of regeneration holds the promise of discovering analogous genes within mammals, opening new avenues for regenerative medicine.
In essence, while the prospect of mice with “mini antlers” may raise eyebrows, it unveils a novel perspective on the regenerative capabilities inherent in mammals. Whether within our own genomes or with a nudge from antler stem cells, the study sparks intriguing possibilities for the future of regenerative medicine.



